Automation Services
As your system-integration partner, Skellig Automation brings our expertise to Life Sciences manufacturing and GMP environments, specializing in DeltaV™, MES, Ignition, and Tulip.
Life Sciences and biopharmaceutical manufacturers are under increasing pressure to launch products faster, modernize, and stay ahead of regulatory demands. Skellig Automation helps our partners overcome these expectations and stay at the cutting edge of the industry.
Any Project, Big or Small
Whether your project has a simple scope for staff augmentation or is a deeply complex capital project, Skellig Automation is ready to bring it to life.
Staff Augmentation
Staff augmentation bridges critical skill gaps across your entire organization, from frontline operations to executive leadership.
Capital Projects
Whether you need highly-specialized expertise or large-project resources, we’re prepared to tackle your capital and fixed fee projects on a timeline that fits your needs.
Consulting Services
DeltaV™, Ignition, MES, MQTT and Tulip implementations tailored to your specific production needs.

Built by Engineers, for Engineers
Life Sciences and biopharmaceutical manufacturers are under increasing pressure to launch products faster, modernize, and stay ahead of regulatory demands. Skellig Automation helps our clients overcome these expectations by strengthening operational reliability, improving compliance, and accelerating technology adoption across new and existing facilities.
Whether your project has a simple scope for staff augmentation or is a deeply complex capital project, Skellig Automation is ready to bring it to life.
Life Sciences Automation Services
Skellig Automation delivers integrated building and process automation services that help Life Sciences and biopharmaceutical manufacturers strengthen both legacy and modern production environments. We support system upgrades, enhancing existing platforms, and optimizing day-to-day performance to create more connected operations.
Our engineers have extensive experience with DeltaV, Ignition, and other common automation technologies. By applying this expertise throughout the system lifecycle, we ensure each solution is functional and capable of evolving with production needs. Our partners gain more dependable operations, reduced reliance on manual processes, and greater predictability.
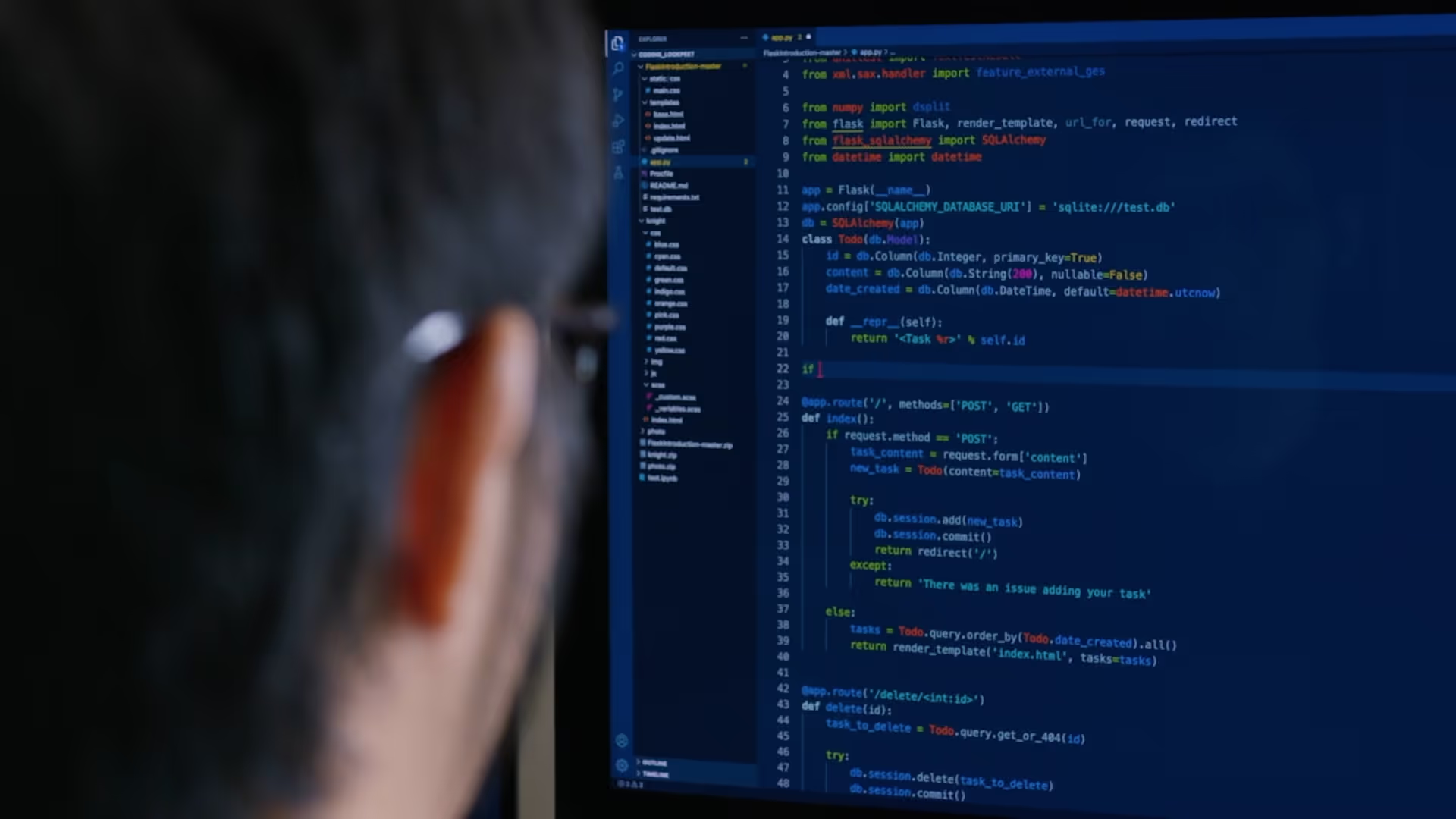

Manufacturing Execution Systems
Skellig Automation supports Life Sciences and biopharmaceutical manufacturers with MES services that strengthen operations across both established and emerging production environments. Our teams work with traditional platforms such as Syncade, PasX, and PharmaSuite as well as modern composable systems like Tulip and Apprentice Tempo, ensuring each implementation fits seamlessly into the broader automation ecosystem rather than functioning as an isolated application.
Our engineers combine the experience required to configure complex MES architectures with a strong understanding of how manufacturing processes actually run. This allows us to align system functionality with operational needs, improve production visibility, and remove inefficiencies. Whether replacing paper-based workflows or delivering a full greenfield deployment, we focus on giving operators a system that supports right-first-time execution and drives measurable performance gains across the facility.
Digital Transformation
Skellig Automation supports manufacturers as they work toward digitally-integrated operations that strengthen both compliance and performance. More than deploying new tools, digital transformation requires a multi-pronged approach that advances digital maturity and supports a robust GMP digital strategy across facility, process, and enterprise systems. Our engineers partner with our clients to ensure each initiative contributes to an integrated and sustainable operational model.
As a partner to leading global pharmaceutical companies, we provide digital transformation support for greenfield sites, major expansions, and modernization efforts within existing facilities. We also offer a dedicated digital transformation assessment, including current system reviews by one of our digital transformation SMEs, identification of obstacles to digital maturity, and a scalable roadmap aligned to the client’s GMP digital strategy. When organizations choose to act on that roadmap, our engineering teams can implement the roadmap with an emphasis on reliability, maintainability, and measurable impact.

Additional Services
.avif)
Manufacturing Intelligence
As companies navigate their digital transformation journeys, they have many data management options. We guide clients through these decisions by assessing current needs, future goals, and the level of digital maturity required to support insight-driven manufacturing. Our engineers work with established time-series data platforms such as Canary and PI as well as cloud databases like Snowflake to create architectures that support integrated operations strategies. We also collaborate with leading Industrial DataOps providers such as HighByte to support a unified namespace or enterprise data warehouse.
Our approach to manufacturing intelligence has been recognized across the industry, including an Ignition Firebrand Award in 2023 for our work at the Center for Breakthrough Medicines.
Validation Services
Validation is not just a compliance exercise, but a means to strengthen operational reliability, reduce risk, and support manufacturing performance. Our validation teams apply a disciplined, practical approach throughout the system lifecycle and beyond, integrating regulatory requirements and industry standards including 21 CFR Part 11, S88, and S95, as well as alarm management best practices.
Our validation professionals have experience throughout the project lifecycle. We help organizations optimize their validation methodologies, improve documentation practices, and enhance confidence in both new and existing systems. Validation is not just a compliance exercise, but a means to strengthen operational reliability, reduce risk, and support sustainable, scalable manufacturing performance.
Ready to Transform Your Operation?
Let's discuss how Skellig Automation can strengthen your manufacturing capabilities.



